NMN and AKG prevent obesity
[summation]
For ' adipocyte differentiation ' that helps prevent obesity, ' upregulation of NAD+ synthesis ' is required, and ' AKG promotes appropriate lipogenesis ' to prevent diet-induced obesity.
This study suggests that NMN+AKG may be a potential treatment for obesity-related metabolic disorders.
0. Differentiation of preadipocytes
In general, obesity is caused by the accumulation of fat cells in tissues due to hypertrophy and hyperplasia of fat cells that occur during the differentiation of preadipocytes and lipid synthesis .
Okabe and colleagues at the University of Toyama in Japan published an article in the journal Frontiers in Cell and Developmental Biology showing that enhanced NAD+ synthesis is important for the development of mature fat.
The expression of PPAR-γ is known to be involved in the differentiation of preadipocytes into adipocytes, which occurs during the differentiation process of preadipocytes. Therefore, studying the regulation of adipocyte differentiation using physiologically active substances is crucial for controlling the development of obesity and obesity-related metabolic diseases.
* PPAR-γ: A major regulator of fat differentiation that exists in fat cells and, when combined with an agonist, activates various genes involved in lipid metabolism and improves insulin resistance and lipid metabolism.
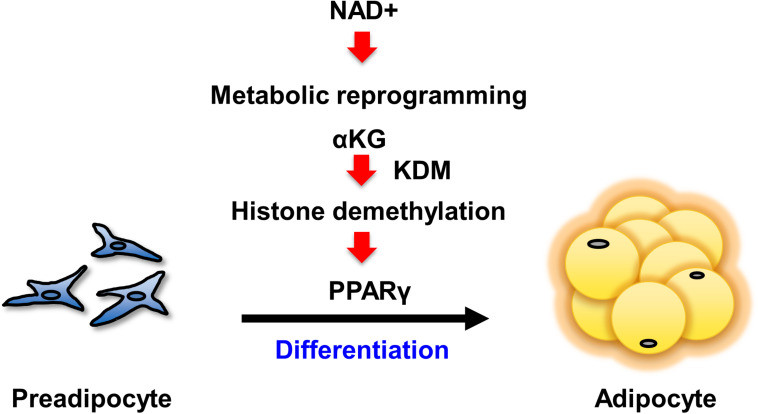
1. What is the role of NAD+ in fat accumulation?
Adipogenesis (maturation of fat cells) is a precisely controlled process. Preadipocytes are cells that have not differentiated into adipocytes, and when adipocytes are derived from preadipocytes, this process is regulated by an elaborate network of transcription factors that coordinate the expression of hundreds of proteins. Regulation by transcription factors When preadipocyte differentiation is disrupted, adipocytes swell and ectopic lipid accumulation occurs, leading to inflammation and metabolic dysfunction .
2. Upregulation of NAD+ synthesis is required for preadipocyte differentiation!
Okabe and colleagues studied the metabolic changes that occur during preadipocyte differentiation. They found that levels of NAD+ and the NAD+ precursor NMN significantly increased during preadipocyte differentiation , accompanied by increases in the levels of enzymes that synthesize both NAD+ and NMN. These data suggest that NAD+ synthesis is upregulated during preadipocyte differentiation .
Next, the researchers investigated whether increased NAD+ levels were necessary for preadipocyte differentiation. They blocked NAD+ production by inhibiting the enzyme that synthesizes NMN during preadipocyte differentiation. Furthermore, suppressing the increase in NMN and NAD+ levels after preadipocyte differentiation was induced significantly reduced preadipocyte differentiation . Furthermore, supplementing differentiated preadipocytes with NMN completely restored NAD+ levels and preadipocyte differentiation.
This demonstrates that elevated NAD+ levels are required for preadipocyte differentiation .
3. The genetic program for adipocyte maturation is regulated by NAD+ synthesis!
Okabe and colleagues investigated whether adipocyte maturation is affected by NAD+ levels. When NAD+ levels were reduced, PPARγ, a key regulator of adipogenesis, and target adipogenic genes were significantly suppressed.
These results indicate that NAD+ must be upregulated to induce PPARγ and that NAD+ metabolism is involved in PPARγ induction during adipogenesis.
4. AKG regulates PPARγ activation in preadipocyte differentiation!
The researchers found that when demethylation of the PPARγ DNA sequence was inhibited, PPARγ activation and preadipocyte differentiation levels were suppressed. Therefore, demethylation of the PPARγ DNA sequence is required for PPARγ activation.
* Demethylation: a chemical process that removes a methyl group (–CH3) from a molecule
Additionally, the metabolite AKG is known to be a cofactor for demethylating enzymes. AKG levels significantly increased during preadipocyte differentiation, correlating with increases in NAD+ levels. Conversely, a decrease in NAD+ levels suppressed the increase in AKG levels after preadipocyte differentiation was induced.
The researchers wanted to determine if there was a link between NAD+, AKG, and adipogenesis. To do this, they supplemented preadipocytes with AKG. AKG induced PPARγ levels and preadipocyte differentiation, both of which were suppressed by inhibiting NAD+ synthesis .
This demonstrates that increased AKG levels by NAD+ are required for demethylation of the PPARγ gene, leading to preadipocyte differentiation.
5. AKG promotes proper fat production, preventing diet-induced obesity!
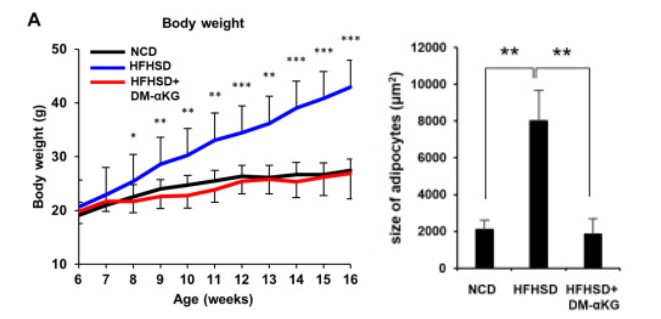
Finally, the researchers studied how NAD+ and AKG affect obesity. They administered AKG orally in the drinking water to mice fed a high-fat, high-sugar diet, which induces obesity. Surprisingly, AKG administration completely suppressed diet-induced obesity, achieving results comparable to those observed in mice fed a normal diet .
Next, the researchers examined the physical properties of the adipose tissue of these mice. While the non-AKG-treated group's fat cells swelled due to the obesogenic diet, the AKG-treated group's fat cells shrank significantly. Furthermore, mice fed the obesogenic diet showed impaired gene expression of the fat-producing program, but this was restored by AKG administration.
Supplementation with AKG prevents diet-induced obesity in mice by promoting appropriate adipogenesis.
The graph on the left shows the weight changes of mice fed different diets over 16 weeks. Mice fed a regular diet (NCD, black) gained significantly less weight than those fed a high-fat, high-sugar diet (HFHSD, blue). In some mice fed a high-fat, high-sugar diet, supplementing their drinking water with AKG (HFHSD+DM-αKG, red) prevented weight gain.
Additionally, in the table on the right, the researchers examined the size of adipocytes in adipose tissue. While a high-fat, high-sugar diet induced swelling of adipocytes, the adipocyte size in the AKG group was significantly smaller than in the high-fat, high-sugar diet group.
6. Can NMN or AKG prevent obesity in humans?
In conclusion, this study suggests that NMN+AKG is a potential therapeutic target for obesity-related metabolic disorders. However, further studies are needed to determine whether these effects can be replicated in humans.
[References and Sources]
[1] Study Zeroes In On NAD+ Metabolism and α-Ketoglutarate to Combat Obesity : https://www.nmn.com/news/nmn-novel-therapeutic-approach-combat-obesity
#nmnobesity #nmnfatcells #akgobesity #akgfatcells #akgefficacy #rockitamerica